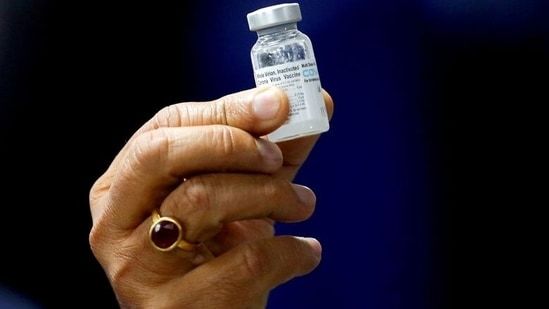
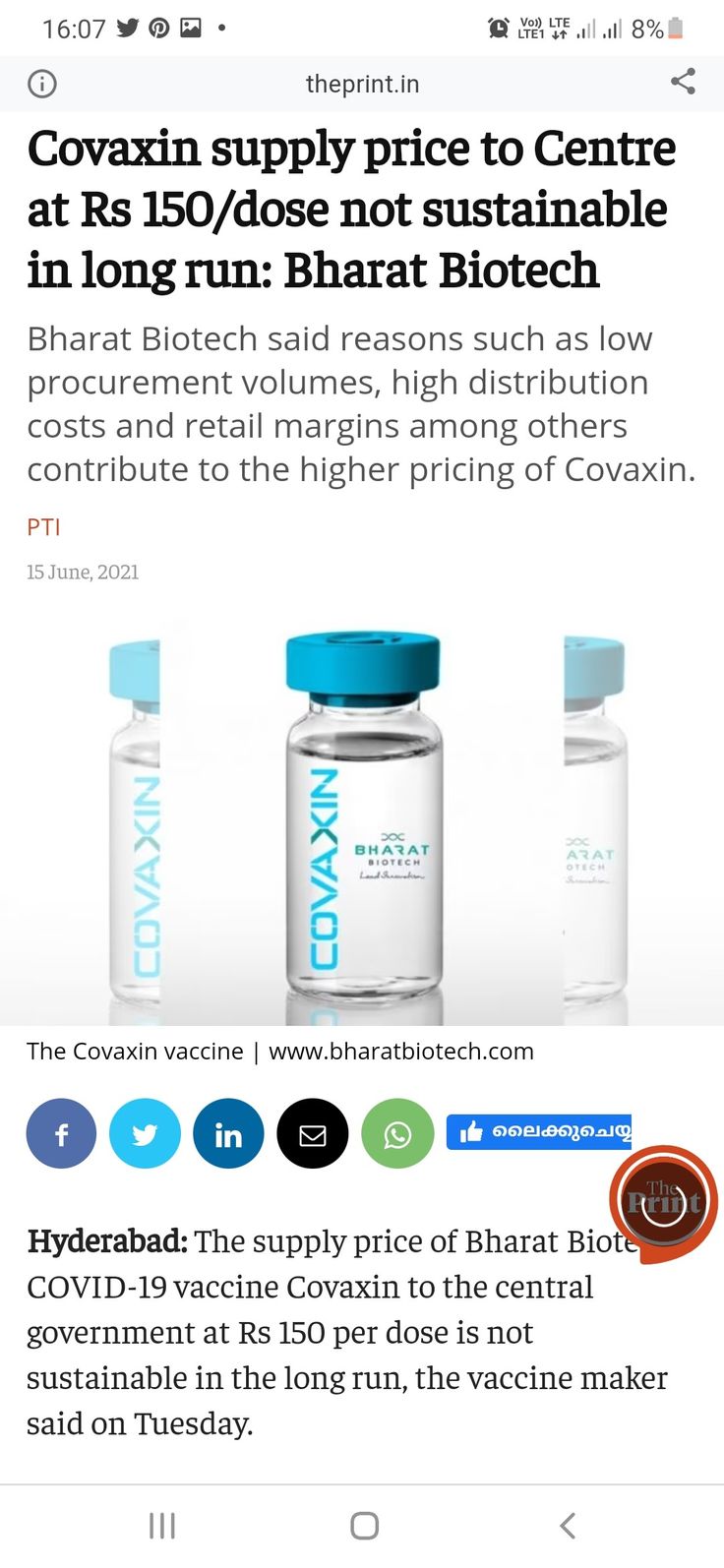
Covaxin
The Emergency Use Listing nod to Bharat Biotechs Covaxin which was found to have 78 per cent efficacy against COVID-19 of any severity expands. It is the second-most widely used coronavirus vaccine in India.

Approved Phase 3 Trials Utkal Today Trials Clinical Trials Medical Research
In June Brazilian.

Covaxin. On Tuesday Guyana recognised Bharat Biotechs Covaxin Indias first indigenous vaccine against Covid-19. Recommended age groups intervals between doses specific groups such as pregnant and lactating women. Indias first indigenous COVID-19 vaccine Bharat Biotechs Covaxin was granted Emergency Use Listing EUL by the World Health Organization WHO on Wednesday.
This comes a day after Covaxin. Travellers who have taken both doses of Bharat Biotechs Covid-19 vaccine Covaxin will be allowed to enter the US from November 8 new agency ANI reported. Covaxin was at the heart of a corruption scandal in Brazil which led to an inquiry into Bharat Biotechs 324 million contract with the Jair Bolsonaro government.
A The World Health Organisation WHO granted its. WHO giving emergency use authorisation to Covaxin would facilitate. Kamala Thiagarajan examines what we know so far.
International Travel Update. Covaxin was also reviewed on 5 October by WHOs Strategic Advisory Group of Experts on Immunization SAGE which formulates vaccine specific policies and recommendations for vaccines use in populations ie. Covaxin has shown 778 effectiveness against the disease and 652 protection against the new highly transmissible delta variant.
After the WHO granted an emergency use listing to Covaxin the United States will allow travellers vaccinated with Bharat Biotechs Covaxin to enter the country from November 8. Covaxin is a COVID-19 vaccine developed by Bharat Biotech an Indian biotechnology company and the Indian Council of Medical Research. 4 hours 48 min ago IST Rome Italy October 31 ANI.
Several health experts have raised concerns over the delay in the grant of Emergency Use Listing EUL for Bharat Biotechs Covaxin by the World Health Organization and sought to know why it is getting stuck. Scroll down for more detailsAlso Read - Kailash. Covaxin is being manufactured in three different locations in India with the current production at over 50 million doses per month.
WHOs final nod to COVAXIN will now enable travellers fully vaccinated with Covaxin to travel to the United States from November 8. While the phase 3 trials entailed 25800 participants between 18-98 years of age including 10 over the age of 60 the analysis was conducted 14 days after administering the second dose of the. Covaxin is a Whole Virion Inactivated Coronavirus Vaccine developed by Bharat Biotech in collaboration with the Indian Council of Medical Research ICMR and the National Institute of Virology NIV.
The company has said it is. World Health Organisation giving approval for Emergency Use. The Indian Council of Medical Research ICMR which collaborated in developing the Covid-19 vaccine said the issue is between the WHO and Bharat Biotech.
Is hoping the World Health Organization will approve the COVID-19 shot Covaxin. Covaxin produced by Indian biotechnology company Bharat Biotech and the Indian governments Council of Medical Research was found to be. Indians await WHO nod for homegrown Covaxin shot to travel abroad.
If the Covaxin approval does not come I will take the risk of going and taking a Saudi-approved vaccine he added sitting in his spacious two-storey house fronted by paddy fields. India has rapidly approved and rolled out Covaxin its own covid-19 vaccine. Millions complain of travel struggles as the vaccine has not been recognised for international travel by several countries.
Covaxin was developed by Indian pharmaceutical company Bharat Biotech in collaboration with the Indian Council of Medical Research a government funded biomedical research institute and its subsidiary the National Institute of Virology. Stuck in a village for nine months and unable to return to his job in Saudi Arabia Sugathan PR.

259 Likes 1 Comments Ias Academy Mohali Chandigarh Iasmagnus On Instagram Download Magnus Institute App For Exam Study Materials Syllabus Exam

ವ ಯ ಕ ಸ ನ ವ ರ Tv9 Reveals Shocking Details Of Covaxin Vs Covishield E In 2021 Reveal Hand Soap Bottle Soap Bottle

Indilivenews Who Should Not Get Covaxin Bharat Biotech Issues In 2021 Fact Sheet Facts News India

Covaxin Water Bottle Bottle Reusable Water Bottle

Covaxin By Bharat Biotech Indian Stock Market Hot Tips Picks In Shares Of India Stock Market Money Plan Day Trading

Bharat Biotech द व र त य र Covaxin क ह य मन ट र यल क म ज र Current Affairs For Cse Ias Youtube Affair Current Youtube

Agonist Molecule Synthesis For Covaxin By Csir Iict In 2021 Agonist Molecules Reaction Types

Andhra Pradesh Receives 4 Lakh Dose Of Covaxin Techgraph In 2021 Consent Forms Andhra Pradesh Dose

Bharat Biotech Covaxin Gets Global Recognition No Serious Side Effects Of Covaxin Said Lancet Youtube In 2021 Recognition Seriously Global
Bharat Biotech Proudly Announces The Animal Study Results Of Covaxin Animal Study Study Animals

ವ ಯ ಕ ಸ ನ ವ ರ Tv9 Reveals Shocking Details Of Covaxin Vs Covishield E In 2021 Reveal Pill Detail